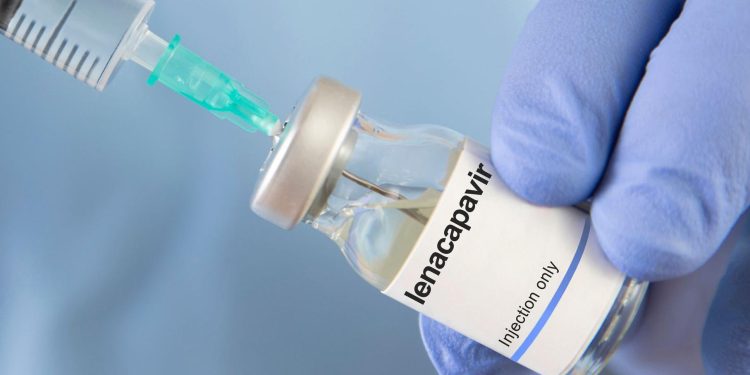
Uganda’s National Drug Authority (NDA) has approved Lenacapavir, a twice-yearly injectable Pre-Exposure Prophylaxis (PrEP) drug manufactured by the US-based company Gilead.
The approval marks a significant milestone in the country’s fight against HIV/AIDS, particularly for individuals at high risk of infection.
Health experts say the long-acting PrEP could improve adherence compared to daily oral tablets, providing sustained protection and reducing new HIV infections.
“This is a game-changer for HIV prevention and a critical step towards ending AIDS by 2030,” officials from the NDA said.
Lenacapavir, administered twice a year, offers a more convenient option for HIV prevention, potentially transforming the prevention landscape for populations vulnerable to the virus.
The approval aligns with Uganda’s ongoing efforts to scale up access to innovative HIV prevention tools and strengthen the country’s commitment to achieving the UNAIDS 95-95-95 targets and ultimately ending the AIDS epidemic.